Publications
2006
2005
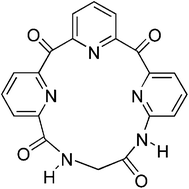
The synthesis and binding properties of a new macrocycle is reported. The host, comprised of three basic pyridines, four hydrogen bond accepting carbonyls, and two hydrogen bond donating amide groups, binds mono-alkyl ammonium salts in a manner that is dependent on the counter-ion of the ammonium guest.
2004
2003
The synthesis of three different nanoscale molecular hosts is reported. These cavitands each possess a highly preorganized cavity with an open portal (nearly 1 nm wide), by which guests can enter and egress the cavity. Additionally, these hosts are deep-functionalized with a crown of weakly acidic benzal C--H groups which can form a variety of noncovalent interactions with guest molecules residing within the cavity. Thirty-one guests were examined for their propensity to form complexes with the hosts. Guests that possess halogen atoms were the strongest binders, suggesting the formation of polydentate C--H⋅⋅⋅X--R hydrogen bonds with the deep crown of benzal hydrogens. Exchange rates between the free and bound states were noted to be dependent on the size of the guest and the solvent used to study complexation. In general, stronger binding and slower exchange were noted for complexations carried out in DMSO with highly complementary guests. The orientation of each guest within the cavity was determined using either EXSY NMR spectroscopy or 1H NMR shift data. Cumulatively these results showed that the principal factors directing orientation were interactions with the benzal groups and the type of solvent. Van't Hoff analyses of selected complexations were also carried out. As well as revealing that all complexations were entropically unfavorable, these experiments provided support for guest orientation determinations, and gave an estimation that the formation of a C--H⋅⋅⋅I--R hydrogen bond releases between 1 and 1.5 kcal mol−1.