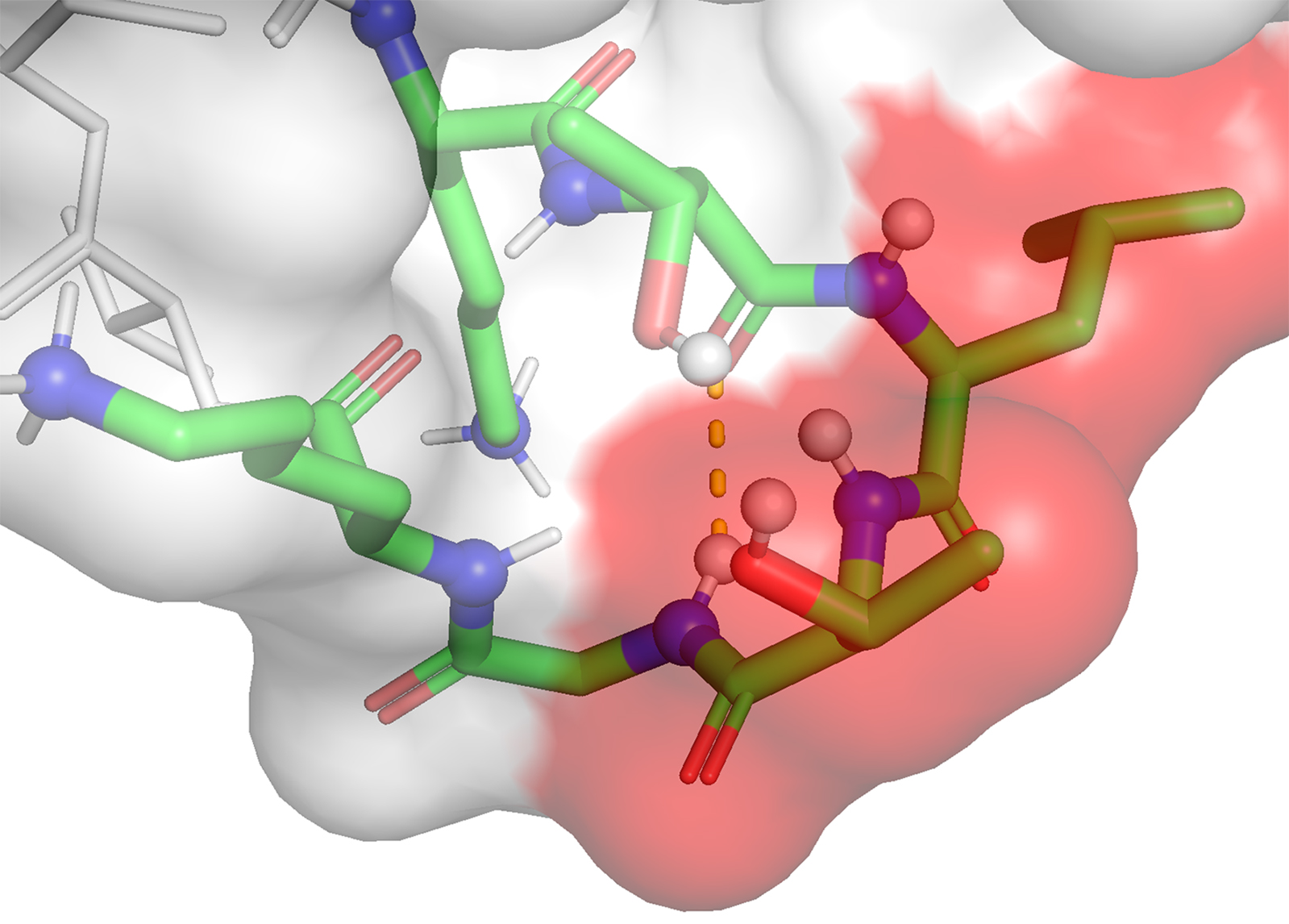
 Another major program of research in the group focuses on peptide/protein non-covalent interactions fundamental to biochemistry and, correspondingly, biologics development. There are large knowledge-gaps in our understanding of these interactions and their effects; gaps that circumscribe advancements in these and related fields.
Another major program of research in the group focuses on peptide/protein non-covalent interactions fundamental to biochemistry and, correspondingly, biologics development. There are large knowledge-gaps in our understanding of these interactions and their effects; gaps that circumscribe advancements in these and related fields.
We use peptides and small proteins as testbeds to examine how salts and buffers interact with these bio(macro)molecules, and so explore how these small solutes affect the secondary and tertiary structure of proteins and correspondingly their downstream physicochemical properties. For these studies we use a variety of peptides including intrinsically disordered (N-terminal) peptides from amyloid proteins and well-folded β-hairpins, as well as the small protein Ubiquitin. This work relies on 2D/3D heteronuclear NMR spectroscopy, differential scanning calorimetry, dynamic and static light scattering, and CD spectroscopy. The work also relies on extensive ab initio calculations and MD simulations.