Dr. Bruce C. Gibb

Professor Bruce C. Gibb, FRSC, was born in 1965 and hails from Aberdeen, Scotland. He received both his B.Sc. and Ph.D. degrees from Robert Gordon’s University, and carried out post-doctoral research with Prof. John Sherman at the University of British Columbia and Prof. James Canary at New York University. His independent career began in 1996 at the University of New Orleans, where he rose through the ranks to become University Research Professor in 2007. At the beginning of 2012 he moved to Tulane University. Prof. Gibb’s over-arching research theme is aqueous supramolecular chemistry; in particular how non-covalent interactions contribute to the hydrophobic effect and the Hofmeister effects, and how these can be harnessed to engender novel molecular compartmentalization phenomena. He serves the supramolecular community as Editor-in-Chief of Supramolecular Chemistry (Taylor & Francis), and as co-founder of the Aqueous Supramolecular Chemistry Workshop series of meetings that have ran since 2015. Since 2017 he has also served the community as chairperson of the advisory committee for the International Conference on Calixarenes (a biannual meeting running since 1991), and as custodian for the C. David Gutsche Award for outstanding contributions to the field of calixarenes. Dr. Gibb serves the greater chemistry community as a quarterly essayist for Nature Chemistry, where he has put pen to paper since issue 1 in 2009.
Research Summary
Aqueous supramolecular chemistry is a highly interdisciplinary research area lying at the interface of organic, physical, and biochemistry. In the Gibb group we study aqueous supramolecular chemistry in two principal ways: 1) we synthesize cavitand hosts to study their solvation, their guest-binding, and their self-assembly into supramolecular capsules; 2) we study peptides and small proteins to examine how they interact non-covalently with buffers, salts, and other biomacromolecules.
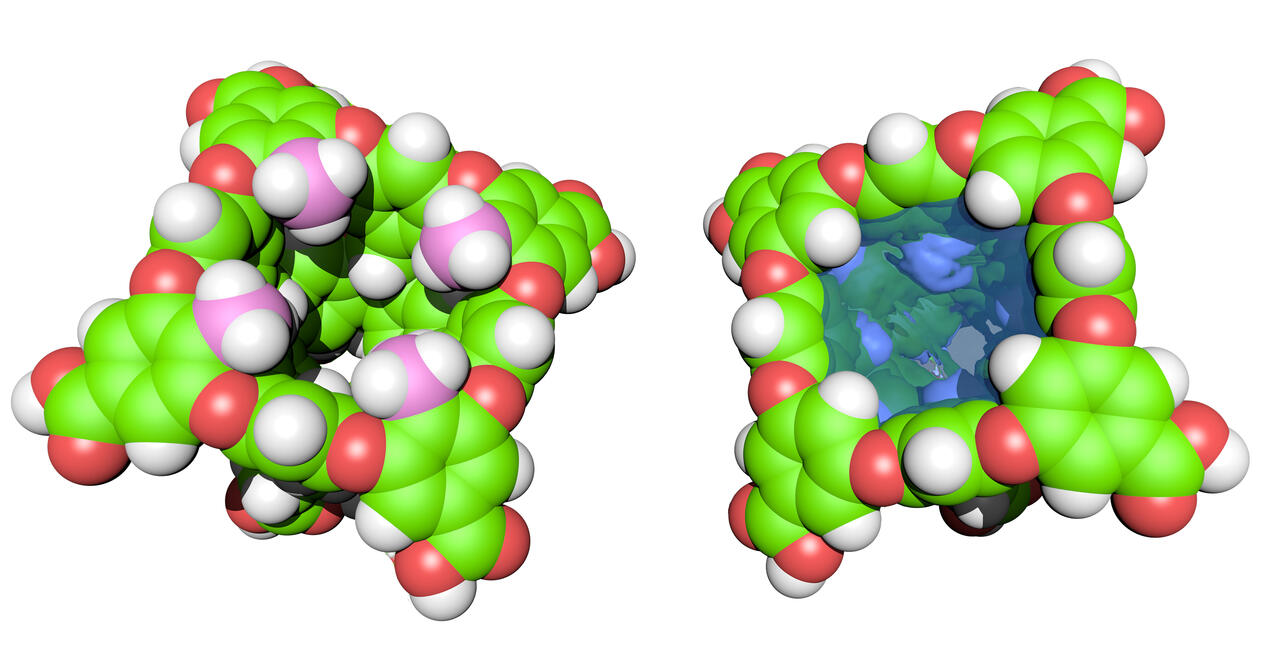
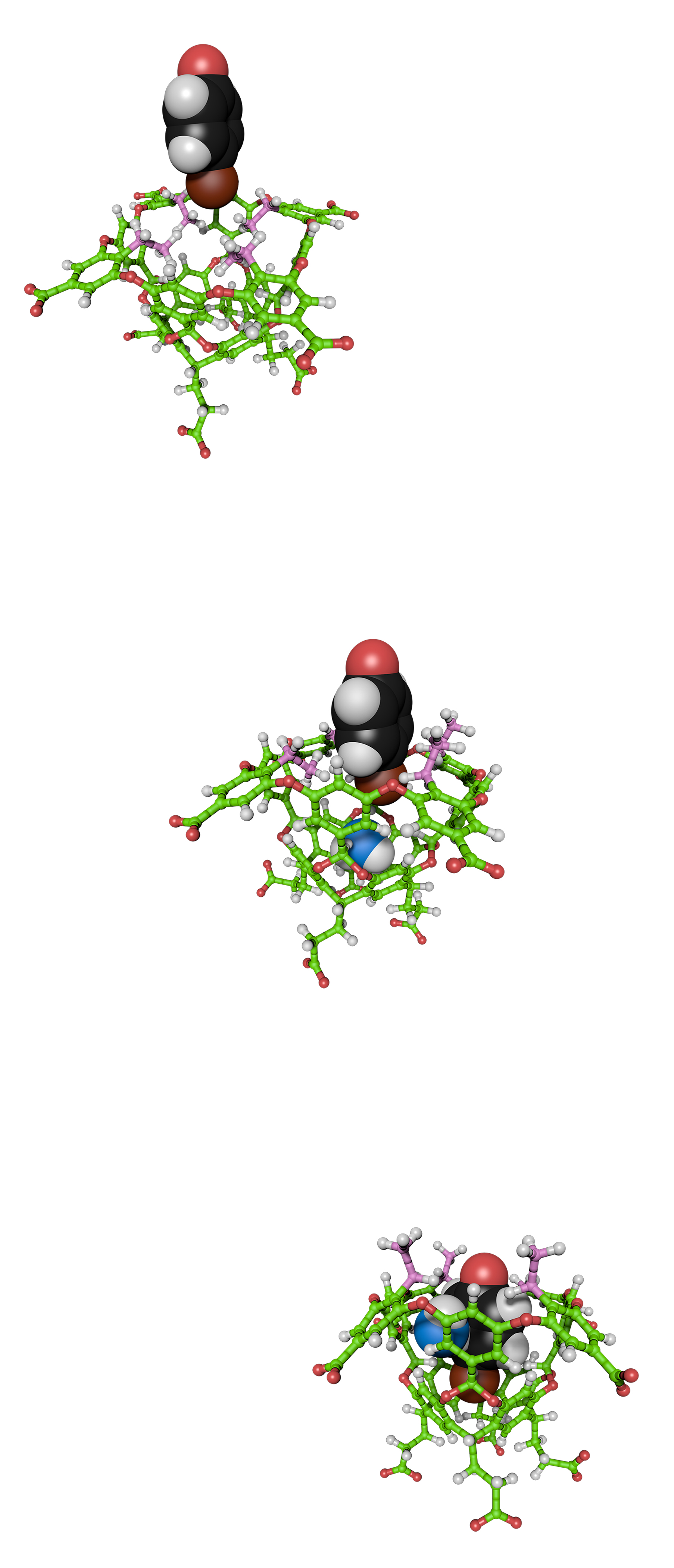
Our cavitand hosts provide valuable information about the context-dependency of the solvation of non-polar pockets, and the ability of ions to shed part of their solvation shell and form non-covalent contacts with organic hosts. Lessons learned from these studies inform our research into the self-assembly of cavitands into yoctoliter (10–26 L) containers, which we use to develop water-based reaction vessels, catalysts, and molecular separators.
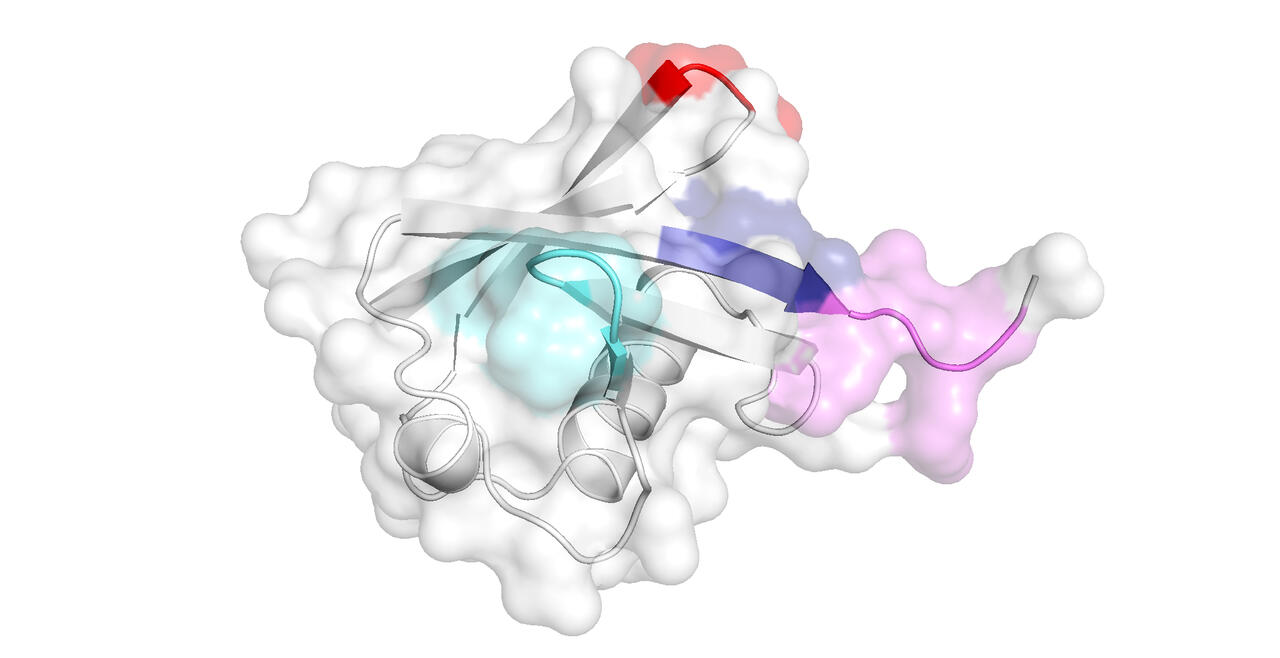
Our research with peptides and small proteins is focused on their interactions with salts and buffers, and how these associations affect their secondary and tertiary structure, and hence their downstream physicochemical properties such as solubility. By such studies we hope to inform biologics development, protein crystallography, and studies into amyloidogenesis.
For analyses we principally utilize NMR spectroscopy, isothermal titration/differential scanning calorimetry, and well as dynamic/static-light scattering. The group also makes extensive use of computational techniques, including ab initio calculations and MD simulations in explicit water. This range of tools, in conjunction with other spectroscopic techniques such as UV-vis, fluorescence, and Raman, provide valuable information regarding the hydrophobic and Hofmeister effects.
Our Interests and Goals
The long-term goals of our ongoing projects include:
- Studying the properties of aqueous-based, supramolecular nano-capsules to identify new and unusual physicochemical phenomena arising through compartmentalization
- Studying the complexation of hosts and guests in aqueous solution to reveal details of the hydrophobic and Hofmeister effects
- Studying the supramolecular properties of peptides and small proteins